Md. Health Agencies Scramble to Pull J&J Vaccines; Vax Equity Initiative Coming to Baltimore

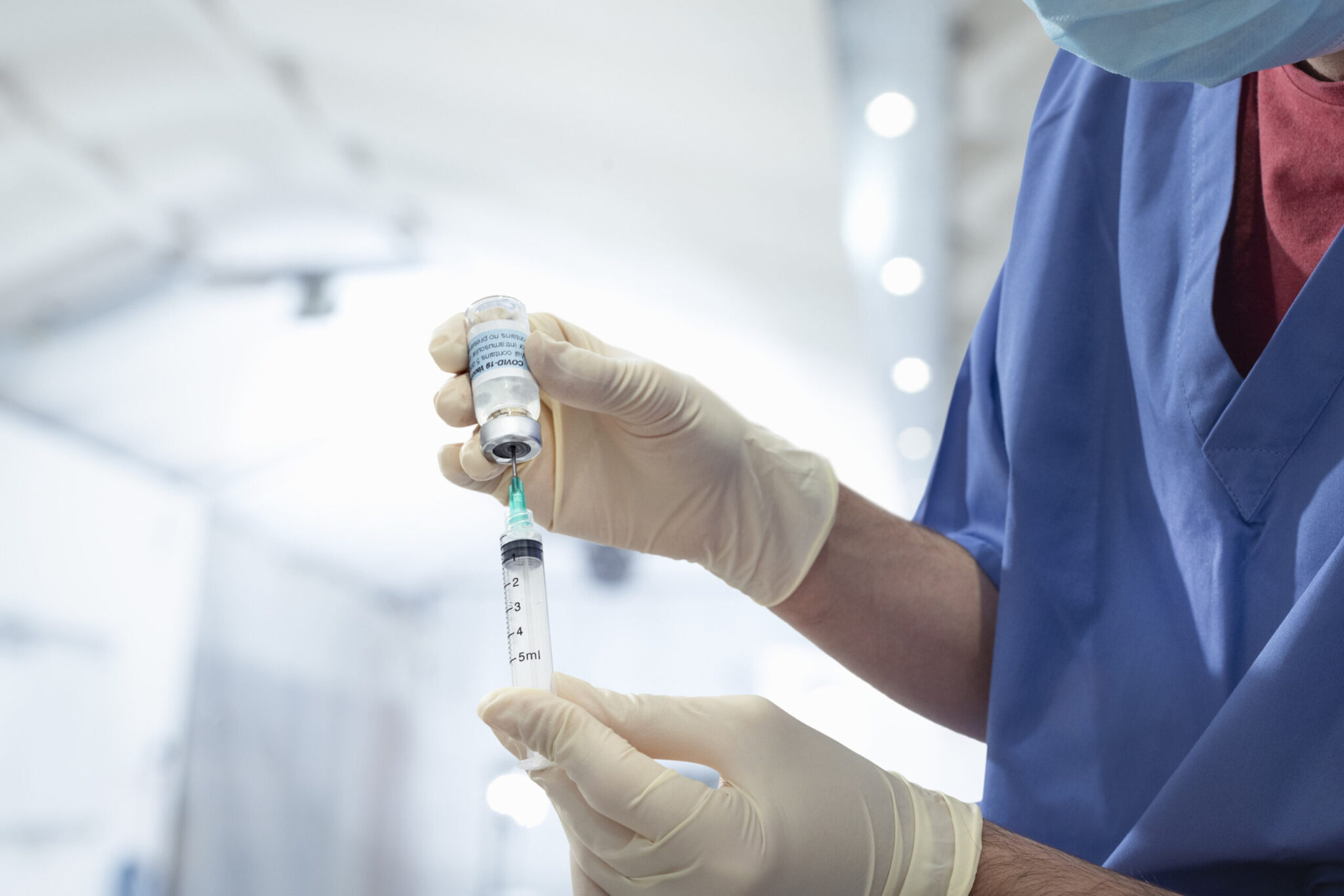
Maryland health departments are canceling Johnson & Johnson COVID-19 vaccine appointments, after the Centers for Disease Control and Prevention and the Food and Drug Administration’s recommendation that it be put on hold because of safety concerns.
After six reported cases of a rare and severe type of blood clot in people who received the J&J vaccine, the FDA and CDC recommended pausing clinics that are administering it.
Officials with the Food & Drug Administration and the Centers for Disease Control and Prevention said in a media briefing that the occurrence of the symptoms was extremely rare. They said the six cases of the blood-clot condition, out of 6.8 million doses administered, have all involved women between the ages of 18 and 48, and that their symptoms occurred six to 13 days after vaccination.
One woman has died, and another is in critical condition.
Halting additional doses will allow officials to alert health care providers of the potential side effects they may see arise in patients, they said, noting that the blood-clot condition requires a less-common treatment to be resolved safely.
“It was clear to us that we needed to alert the public,” said Anne Schuchat, the CDC’s principal deputy director, adding that the pause will allow the health care community to “learn what they needed to learn about how to diagnose, treat and report” on adverse reactions.
The Maryland Department of Health has directed all vaccine providers to stop giving out the J&J vaccine until further notice.
“Based on the federal government’s recommendation and out of an abundance of caution, the Maryland Department of Health directs all Maryland COVID-19 vaccine providers to pause the administration of Johnson & Johnson COVID-19 vaccines until further federal guidance is issued,” the department said in a statement.
They added that providers should continue to maintain their supplies of the J&J vaccines in a manner that prevents waste.
Montgomery County’s health officer, Dr. Travis Gayles, said the county was already dealing with a shortage of vaccines due to the earlier reduction of federal allotments to the states.
In the short term, Gayles said, the operations of the mass vaccine site in Germantown should not be affected by the decision to pause the use of Johnson & Johnson vaccines.
“We had fully expected to be using primarily Pfizer at that site beginning tomorrow,” Gayles said Tuesday during a Montgomery County Council session while officials monitored a public call with the FDA.
Council members worried the news would increase the skepticism some have about the coronavirus vaccines.
“There was already a high degree of misinformation out there,” Councilmember Gabe Albornoz (D) said, “and already I’m beginning to see a lot of ‘I-told-you-so’s.’”
Montgomery County had about 960 doses of the J&J vaccine on hand for appointments at the mass vaccination site in Germantown on Tuesday.
The Hagerstown mass vaccination site has switched to providing the Pfizer vaccine on Tuesday and will honor all appointments.
The J&J vaccine has been viewed as a critical part of ramping up the U.S. vaccination effort, because it requires just one dose and has less-stringent storage requirements.
But so far, it’s been a small share of the overall doses administered — the 6.8 million doses given as of Monday are among nearly 190 million nationally, according to the CDC’s vaccine tracker. Another 9 million have been shipped to states but not yet administered.
In comparison, 98 million doses of the Pfizer vaccine and 85 million of the Moderna vaccine have been administered, both of which require two shots given weeks apart. The rare blood-clot condition has not been reported following doses of those shots.
Johnson & Johnson has faced challenges in scaling up its vaccine production, and had to discard 15 million doses due to errors at a Maryland manufacturing facility.
The federal announcement comes as demand for vaccines is increasing as states remove eligibility requirements, acting on directions from the Biden administration to make every American eligible for a vaccine by Monday.
Jeff Zients, the White House’s COVID-19 response coordinator, said Tuesday that there is enough supply available of Pfizer and Moderna doses to continue the current pace of 3 million shots per day.
He added that the Biden administration is working with states and federally run vaccine sites to reschedule anyone with a J&J appointment to receive one of the other available shots.
It’s not yet clear how long the pause on J&J doses will last. The FDA’s independent vaccine advisory panel, which authorized each of the three current vaccines, is set to meet on Wednesday to review the cases that led to the federal recommendation.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, estimated that based on what he’s heard from other top public health officials, the review is more likely to last “days to weeks,” rather than “weeks to months.”
Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said the pause is “a recommendation” and “not a mandate,” adding that federal officials would not stop a provider from administering the vaccine if a provider and a patient determine that the benefits outweigh the risks.
But most Americans are not getting their vaccine directly from a primary-care provider, and instead are going through federally or state-run vaccination sites, or local pharmacies receiving doses through the government.
The blood-clot condition, known as cerebral venous sinus thrombosis, had combined with low levels of blood platelets in the six cases under scrutiny according to the FDA.
Anyone who has received a J&J vaccine and develops severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider, FDA officials said.
National vaccine equity initiative coming to Baltimore
While the state scrambles with potential vaccine shortfalls due to the temporary suspension of the J&J vaccine, the Rockefeller Foundation announced Tuesday that it has selected Baltimore as one of five pilot cities to launch a $20 million Equity-First Vaccination Initiative designed to improve vaccination rates among communities of color, which have been disproportionately impacted by the COVID-19 pandemic.
With Rockefeller’s support, Open Society Institute-Baltimore (OSI-Baltimore) will launch the Baltimore Equitable Vaccination Initiative to provide medically sound, culturally competent information about vaccine safety and improve distribution and delivery mechanisms to reach disconnected Baltimoreans through pop-up vaccination sites and mobile clinics across the city.
In addition to on-the-ground support, the initiative will support resource hubs to assist in addressing access to health care, housing, and other critical health-sustaining needs and advocate for systemic change that will address community infrastructure and social service needs to reduce health disparities.
“The Rockefeller Foundation has been a key ally to Baltimore and OSI from the early days of COVID-19 when it helped to create Baltimore Health Corps, an initiative to recruit, train, and employ more than 300 residents who were jobless due the pandemic as contact tracers,” said Danielle Torain, director of Open Society Institute-Baltimore. “We are proud to continue this partnership through the Baltimore Equitable Vaccination Initiative, which will ensure that communities have equitable access to the vaccines, information, and other resources.”
OSI-Baltimore will partner with a number of local, community-based organizations and government agencies to ensure that disproportionately impacted communities, including Black, Indigenous and people of color (BIPOC) and low-wage essential workers, are adequately served.
“Because of existing structural inequalities—including health care access, wealth gaps and systematic racism—people of color have been much more likely to both contract Covid-19 and die from this virus,” said Otis Rolley, senior vice president for the U.S. Equity and Economic Opportunity Initiative at The Rockefeller Foundation and a former Baltimore City official who is a former member of OSI-Baltimore’s Advisory Board. “The Rockefeller Foundation is launching this initiative because a vaccination strategy that does not seek to directly combat inequities stands to further entrench them.”
Representing less than one-third of the 63 million people who are now fully vaccinated in the United States, communities of color are twice as likely to die from COVID-19 and three times as likely to be hospitalized as white Americans. To close this gap, the Rockefeller Foundation will initially collaborate with five organizations to deploy equity-first, hyper-local public health interventions in five U.S. cities.
Besides Baltimore, they are: Chicago, Houston, Newark, N.J., and Oakland, Calif.
Josh Kurtz of Maryland Matters and Valerie Bonk of WTOP News contributed to this report.

 Creative Commons Attribution
Creative Commons Attribution